 載入中...請稍候...
載入中...請稍候...- 首頁
- 國際認證
國際認證
《美國食品及藥物管理局》(U.S. Food and Drug Administration, FDA)
![]()
《美國食品及藥物管理局》隸屬於美國聯邦政府,其主要職能為負責對美國國內生產及進口的食品、膳食補充劑、藥品、疫苗、生物醫藥制劑、血液制劑、醫學設備、放射性設備、獸藥和化妝品進行監督管理,同時也負責執行公共健康法案,包括公共衛生條件及州際旅行和運輸的檢查、對於諸多產品的安全控制等等。《美國食品及藥物管理局》實施安全監管範圍很廣,例如:對於藥品的監管就涉及它的每一個方面,從藥品測試、制造、標韱規範、廣告、市場營銷、功效直到藥品安全。《美國食品及藥物管理局》尤其對生產商的設備、衛生環境及工作流程有嚴格監管的機制,以確保其生產成品的質量,所以能通過《美國食品及藥物管理局》審查成為獲認證的藥品生產廠房,其產品的質量有高度認受性;而ARIIX產品正正就是由擁有FDA認證的生產廠房中制造的。
 《良好生產規範》 (Good Manufacturing Practice, GMP)
《良好生產規範》 (Good Manufacturing Practice, GMP)
![]()
《良好生產規範》是指對食物、藥品、醫療產品生產和質量管理的法規。為了保持這一認證,這些制造商將被定期檢查和審核,以確保該制造商的人員、制造現場、衛生、設備、工作質量、生產過程控制、倉儲、發貨、和發貨後操作方法均達到最佳標准。《藥品生產質量管理規範》更是藥品級的GMP,它進一步用於藥品生產的全過程,包括原材料挑選,以及生產中影響成品質量的所有關鍵工序。生產商按《藥品生產質量管理規範》在藥品級別的生產規範下進行生產,以確保產品衛生、安全。
![]() 《非處方藥認證》(Over-The-Counter, OTC)
《非處方藥認證》(Over-The-Counter, OTC)
![]()
OTC(Over-The-Counter)藥品,也被稱為非處方藥,是不需要醫生所開的處方就能直接購買使用的藥品。由於消費者可以直接從市場上購買到非處方藥,故此,《美國食品及藥物管理局》對非處方藥及非處方藥制造藥廠的要求非常嚴格,意在保障大眾消費者的健康。
 《美國國家衛生基金會》(National Sanitation Foundation, NSF)
《美國國家衛生基金會》(National Sanitation Foundation, NSF)
![]()
《美國國家衛生基金會》是一個非政府組織/非營利組織(NGO/NPO),在有關公共衛生、公眾安全之標准開發、產品認證、教育及風險管理等領域上扮演著世界領先者的角色。《美國國家衛生基金會》已成為一個具有國際影響力和公信力的機構。獲得《美國國家衛生基金會》認證,需要通過大量的產品測試、原料分析,對生產現場的突擊檢查。生產設備必須符合相關NSF/ANSI標准的要求,評審過程中還包括:生產現場須接受每年的跟蹤審核,這不只是一次性的檢驗活動,為的是確保生產商持續符合標准的每項要求、產品生產遵循品保與品管程序、產品按計劃抽樣及再測試、以及產品標簽和宣傳資料真實准確。
1. 概況
* Ariix的產品質量達到了美國甚至國際營養補充品的最高級。據最近2014.10在香港召開的Ariix全球年會公布,Ariix的全衡營養素在Lyle McWilliam主編、最近一次出版的《北美營養補充品比較指南》獲得最高的五星評價。
* Ariix成為敢做100%功效保証的美國兩家公司之一。
* 中國國家公眾營養改善項目主任、中國大健康聯盟執行理事長于小冬教授在Ariix公司香港全球年會上對Ariix公司高度評價,高度期待。他說:“我對Ariix公司有一個期望,我期望Ariix公司在不久的將來能成為中國大健康產業發展的領軍企業,我希望你們成為中國政府、中國收費市場都很熟悉、都很熱愛、都離不開的重要企業……我感到我的這個願望一定能實現。你們的事業是光榮的,你們的使命是偉大的,我會堅定地和你們站在一起。” 《中國公眾營養網》 2014.9.11用了標題“國際保健巨擘ARIIX落戶天津空港經濟區 ”
* 其質量保障的秘密是什麼?

重要原因(一)
- FDA嚴格、健全的法律監管:
- FDA對藥品的監管(certified,approval,認証)
- FDA對食品、營養補充品的監管(registered,注冊,登記)
* 美國食品藥品管理局FDA隸屬於美國聯邦政府健康與人類服務部(U.S. Department of Health and Human Services),其主要職能是負責對美國國內生產及進口的食品、膳食補充劑、藥品和化妝品進行監督管理。對膳食補充劑Dietary Supplement的監管部門是FDA的食品安全與應用營養中心。營養補充品一旦出現問題,將進行嚴格的追查制度使得FDA具有強大的威懾力。
* FDA不僅在美國有公信力,在世界上許多國家都得到承認。
* 由於FDA的監管力度比較大,美國的藥品和營養品從生產到銷售都有健全的法律和制度保障。
重要原因(二)
* Ariix公司自身對產品質量的追求,是通過其嚴格、多維度的產品質量保障體系來實現的:1、國際認証﹔2、權威的國際獲獎,3、權威媒介收錄等三種形式。
1、國際認証:Ariix共獲得11項國際認証。
從認証機構的屬性來看,有政府機構認証、行業認証、非行業的第三方獨立機構認証﹔
從產品的整個管理過程來看,包括對原材料的認証、產品生產過程的認証、產品成品的認証等﹔
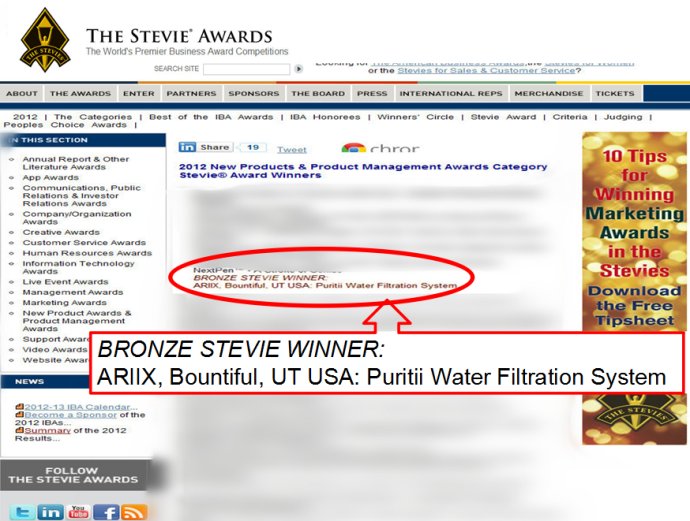
2、權威的國際獲獎:史迪威大獎
3、權威媒介收錄:
Ariix的全衡已進入美國的藥典PDR(美國內科醫生案頭參考:Physician’s Desk Reference)電子版,紙質版的PDR將於明年正式出版﹔
Lyle McWilliam著:《營養補品的比較指導》系列第4、5、6版本的評分。在最近的版本中上升為五顆星的評價。
以上三個部分組成了一個完整、有機、多層次、多維度的質量保障體系。
二、認証分類
Ariix獲得了11種認証,按生產順序分:
- 原材料認証:USDA、非GMO、USP
- 產品制造工廠認証:NSF、GMP、FDA、OTC
- 產品成品認証:NSF、BSCG、ConsumerLab(AL)
- 整體保証:100%功效保証
三、原材料認証 ARIIX國際認証

美國農業部USDA認証
* 美國最權威的有機認証,標准極為苛刻。其具體標准:
* 使用成份不含 任何化學合成物質,如化肥,殺虫劑,抗生素,食品添加劑以及轉基因動植物。
* 使用成份所生長的土壤,至少三年以上沒有使用過化學合成物質。
* 定期檢查生產和銷售記錄。
* 保持有機認証產品的嚴格物理隔離。
* 檢驗有機產品的生產設施廠房
* 全部是由有機成份的產品可以使用標簽注明“100%有機”或使用USDA徽標。
* 使用超過70%有機成份的產品可以稱作“使用有機成份制造”,但不能USDA徽標。
非GMO認証
不含轉基因原料

美國藥典標准:
1、 在體內完全溶解
2、 不含未告知成分
3、 不含污染物
4、 高生物利用率
* “輔酶-奧米加所選用的魚油是美國唯一受美國藥典委員會(USP)認証的魚油。品質及純淨度更超越歐盟、挪威食品安全管理局、加拿大衛生部和美國食品及藥物管理局(FDA)等所訂立的嚴格質量和純度標准。我們的魚油更獲美國食品及藥物管理局(FDA)給予”安全可靠“(GRAS)之檢驗標記”。
* (引自Ariix香港公司:《ARIIX 輔酶-奧米加獲ConsumerLab.com(消費者實驗室)認証》新聞發布會,2014.4.1)
4. 對Ariix產品成品的認証
1、NSF
2、BSCG
3、ConsumerLab:CL

* NSF的產品認証
在NSF網站上就能查到Ariix以下產品通過了NSF認証:
名稱 每天推薦用量
ARIIX minerals 4 Capsules
ARIIX vitamins 6 Capsules
ARIIX vináli™ 2 Capsules

BSCG美國禁藥物組織
* 其認証証明了我們的產品已通過了嚴格的安全性和純度的測試,不含任何有害成分。而在這個行業中隻有少數營養補充劑能獲得此認証,因為這個認証不單成本很高,而且安全檢測標准。採購成分的審查,工廠設施,制造過程以及監控實施非常嚴格。
* Wally Joyner - 專業運動員,美國職棒大聯盟全明星(MLB all-star)這樣說:
“身為一個專業運動員,營養補充劑的質量和安全性是我最大的考慮。很多運動員都避免服用營養補充劑,因為他們害怕發現它們包含了違禁物成分或跟營養補充劑標簽上聲稱的不一樣。
* “獲得BSCG的認可讓我又多一個理由更相信ARIIX。我不單止感覺更好,更有活力,而且通過BSCG認証讓我知道這是市面上最安全的產品。”
* 迪安娜對BSCG禁藥物組織認証視頻
BSCG鏈接:http://www.bscg.org/Certified-Products.php
http://www.bscg.org/Ariix.php

ARIIX 輔酶-奧米加獲ConsumerLab.com 認証:
* “全球首屈一指的健康及保健公司ARIIX 今天(2013.4.1)宣布,其廣受歡迎的營養補充品輔酶-奧米加,從細胞層面開始,促進最佳的心臟和大腦功能,已經由消費者實驗室, 獨立測試並獲得認証。消費者實驗室為一個權威之獨立第三方公司,專門就保健品進行測試,以幫助消費者和醫療保健專業人士安心購買產品。消費者實驗室的認証是ARIIX最新之獨立測試公司和組織發出的認証,同時是ARIIX產品經過嚴格測試的認可。”(引自Ariix香港公司網站)
六、國際獲獎
* 國際企業大獎(The international Business Award)的史迪威獎(Stevie Awards)是被紐約時報譽為“企業界奧斯卡獎”。今年史迪威獎(Stevie Awards)金獎、銀獎和銅獎獲得者是從來自50多個國家的組織和公司提交的3200多項參賽項目中評選出來的。全球所有組織都有資格參與這個國際企業大獎(The international Business Award)的爭奪,來自全球的企業專業人士對參評項目進行了初評。
* 根據平均得分確定了入圍項目,進入最終評選 。由來自全球的近300位高級管理人士所組成的10個專門委員會的委員完成了最終評選 。


* “ARIIX” 來自拉丁文“黃金”(AURUM)的詞根,以此為名,意在制定營養補充品中的黃金標准。我們一開始就致力保持產品的最高質量。每一款產品都必須經過嚴格檢驗,並証實超越行業標准,方能加蓋“Ariix 承諾 100%產品效能保証” (The ARIIX Promise 100% Potency Guarantee)的印章,代表產品標識上所表示的成分與產品實質含量一致,以確保產品能發揮其成分的100%功效。
補充知識
* 美國聯邦政府FDA的GMP
* FDA的GMP又分別有食品和藥品兩個級別。
-- 第一,在食品級別下面,包括一般的食品conventional food 和 營養補充劑Dietary Supplement等兩大類的GMP,其嚴格程度是不同的。
-- 第二個級別是藥品級別的GMP,包括非處方藥OTC Drug和處方藥Prescription Drug兩個級別。
* 其嚴格程度是從食品、膳食補充劑、非處方藥、處方藥,逐漸從嚴格到極其嚴格過渡。
* FDA於2010年專門出台適用於營養補充劑的GMP。參見Guidance for Industry: Current Good Manufacturing Practice in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements; Small Entity Compliance Guide,簡稱DS CGMP。
* DS CGMP對美國國內及進入美國的營養補充品的生產起著法律監管作用。主要是通過要求營養補充品制造公司必須在FDA進行注冊登記,要求各公司自己留存整個制造過程的所有資料、樣品,以備FDA需要的時候能迅速地查看到。這就起到了為美國人民保障食品安全的作用。
* 當然,DS CGMP不是要求強制執行的,而是強調推薦執行。對於質量一般的營養補充品生產公司,FDA的DS CGMP無疑是要求相當高的,但對於高質量的營養補充品生產公司如Ariix來說,其要求又隻是基礎性的,因為還有很多GMP要求比這個更高,如NNFA,NSF的GMP要求會更高。
* What is FDA's role in regulating dietary supplements versus the manufacturer's responsibility for marketing them?
* In October 1994, the Dietary Supplement Health and Education Act (DSHEA) was signed into law by President Clinton. Before this time, dietary supplements were subject to the same regulatory requirements as were other foods. This new law, which amended the Federal Food, Drug, and Cosmetic Act, created a new regulatory framework for the safety and labeling of dietary supplements. Under DSHEA, a firm is responsible for determining that the dietary supplements it manufactures or distributes are safe and that any representations or claims made about them are substantiated by adequate evidence to show that they are not false or misleading. This means that dietary supplements do not need approval from FDA before they are marketed. Except in the case of a new dietary ingredient, where pre-market review for safety data and other information is required by law, a firm does not have to provide FDA with the evidence it relies on to substantiate safety or effectiveness before or after it markets its products. Also, manufacturers need to register themselves pursuant to the Bioterrorism Act with FDA before producing or selling supplements. In June, 2007, FDA published comprehensive regulations for Current Good Manufacturing Practices for those who manufacture, package or hold dietary supplement products. These regulations focus on practices that ensure the identity, purity, quality, strength and composition of dietary supplements. (http://www.fda.gov/Food/DietarySupplements/QADietarySupplements/default.htm)
* Where can I get information about a specific dietary supplement?
* Manufacturers and distributors do not need FDA approval to sell their dietary supplements. This means that FDA does not keep a list of manufacturers, distributors or the dietary supplement products they sell. If you want more detailed information than the label tells you about a specific product, you may contact the manufacturer of that brand directly. The name and address of the manufacturer or distributor can be found on the label of the dietary supplement.
* Who has the responsibility for ensuring that a dietary supplement is safe?
* By law (DSHEA), the manufacturer is responsible for ensuring that its dietary supplement products are safe before they are marketed. Unlike drug products that must be proven safe and effective for their intended use before marketing, there are no provisions in the law for FDA to "approve" dietary supplements for safety or effectiveness before they reach the consumer. Under DSHEA, once the product is marketed, FDA has the responsibility for showing that a dietary supplement is "unsafe," before it can take action to restrict the product's use or removal from the marketplace. However, manufacturers and distributors of dietary supplements must record, investigate and forward to FDA any reports they receive of serious adverse events associated with the use of their products that are reported to them directly. FDA is able to evaluate these reports and any other adverse event information reported directly to us by healthcare providers or consumers to identify early signals that a product may present safety risks to consumers. You can find more information on reporting adverse events associated with the use of dietary supplements at Dietary Supplements - Adverse Event Reporting.
* Do manufacturers or distributors of dietary supplements have to tell FDA or consumers what evidence they have about their product's safety or what evidence they have to back up the claims they are making for them?
* No, except for rules described above that govern "new dietary ingredients," there is no provision under any law or regulation that FDA enforces that requires a firm to disclose to FDA or consumers the information they have about the safety or purported benefits of their dietary supplement products. Likewise, there is no prohibition against them making this information available either to FDA or to their customers. It is up to each firm to set its own policy on disclosure of such information. For more information, see claims that can be made for dietary supplements
* 正因為如此,Ariix自己設置100%功效的黃金標准,並且通過獲得多個第三方認証來証明自己的嚴格標准。
* What is FDA's oversight responsibility for dietary supplements?
* Because dietary supplements are under the "umbrella" of foods, FDA's Center for Food Safety and Applied Nutrition (CFSAN) is responsible for the agency's oversight of these products. FDA's efforts to monitor the marketplace for potential illegal products (that is, products that may be unsafe or make false or misleading claims) include obtaining information from inspections of dietary supplement manufacturers and distributors, the Internet, consumer and trade complaints, occasional laboratory analyses of selected products, and adverse events associated with the use of supplements that are reported to the agency.
* Is it legal to market a dietary supplement product as a treatment or cure for a specific disease or condition?
* No, a product sold as a dietary supplement and promoted on its label or in labeling* as a treatment, prevention or cure for a specific disease or condition would be considered an unapproved--and thus illegal--drug. To maintain the product's status as a dietary supplement, the label and labeling must be consistent with the provisions in the Dietary Supplement Health and Education Act (DSHEA) of 1994.*Labeling refers to the label as well as accompanying material that is used by a manufacturer to promote and market a specific product.
* Why do some supplements have wording (a disclaimer, 免責申明) that says: "This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease"?
* This statement or "disclaimer" is required by law (DSHEA) when a manufacturer makes a structure/function claim on a dietary supplement label. In general, these claims describe the role of a nutrient or dietary ingredient intended to affect the structure or function of the body. The manufacturer is responsible for ensuring the accuracy and truthfulness of these claims; they are not approved by FDA. For this reason, the law says that if a dietary supplement label includes such a claim, it must state in a "disclaimer" that FDA has not evaluated this claim. The disclaimer must also state that this product is not intended to "diagnose, treat, cure or prevent any disease," because only a drug can legally make such a claim.



